We are studying (i) the cellular and molecular basis of immunologic self tolerance and autoimmune disease as its abnormality; (ii) the strategy of eliciting effective immune responses to autologous tumor cells by manipulating the mechanism of immunologic self-tolerance; and (iii) the cause and pathogenetic mechanism of rheumatoid arthritis by analyzing a newly established mouse model of autoimmune arthritis.
Recently projects are as follows.
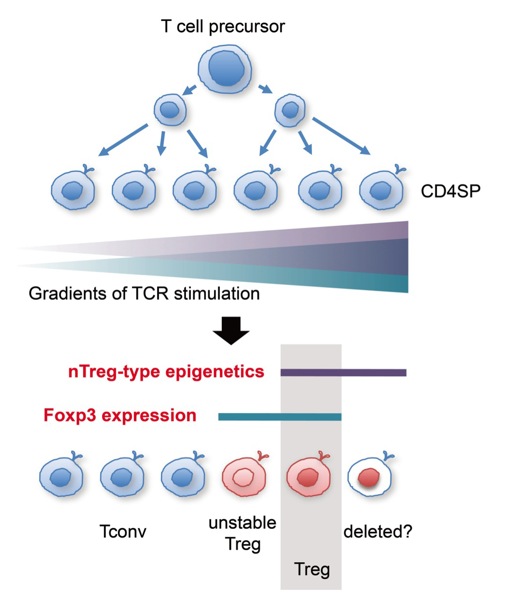
1. Epigenetic conversion is essential for the development of regulatory T cells
The transcription factor Foxp3 is essential for the development of regulatory T (Treg) cells, yet its expression is insufficient for establishing the Treg cell lineage. We showed that Treg development was achieved by the combination of two independent processes, i.e., the expression of Foxp3 and the establishment of Treg-specific CpG hypomethylation pattern. The Treg-type CpG hypomethylation was fully established without Foxp3, and required for Foxp3+ T cells to acquire Treg-type gene expression, lineage stability, and full suppressive activity. Thus, those T cells in which the two events have concurrently occurred are developmentally set into the Treg cell lineage. This model explains how Treg cell fate and plasticity is controlled. We are now trying to clarify the whole epigenetic information of Treg cells, the molecular mechanisms by which Treg-specific CpG hypomethylation is established, and extracellular signals for inducing the hypomethylation. The induction of Treg-specific hypomethylation in T cells would be exploited to generate functionally stable Treg cells for clinical use.